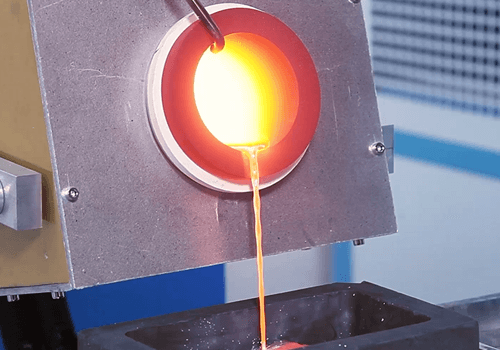
Precious Metal Furnace
Achieve the melting, refining, and casting of gold, silver, platinum, palladium, rhodium, iridium, ruthenium, and other precious metals with SuperbMelt Precious Metal Furnaces.


SuperbMelt precious metal furnace is widely used in jewelry manufacturing, precious metal recycling, and jewelry processing. It enables precise melting, refining, and casting of various precious metals such as gold, silver, platinum, palladium, rhodium, iridium, and ruthenium.
The equipment is designed with highly efficient heating performance, capable of rapidly reaching temperatures up to 2800℃. It can melt 4–12 kg of gold, silver, or platinum within just 2–5 minutes, perfectly meeting the needs of small-batch precious metal processing in jewelry manufacturing and effectively improving production efficiency.
At the same time, the furnace has been fully optimized for safety and stability: the tilting function ensures accurate pouring, while the reliable water-cooling system provides efficient cooling, safeguarding operator safety and maintaining stable equipment performance. With its excellent adaptability to a variety of precious metals, it has become a premium choice in the field of precious metal processing.
| Model number | SPB-TB2 | SPB-TB4 | SPB-TB5 |
| Power source | Three phase 380V, 50/60Hz | Three phase 380V, 50/60Hz | Three phase 380V, 50/60Hz |
| Power | 15 kw | 15 kw | 15 kw |
| Applicable metal | Platinum, palladium, gold, silver, steel, copper and their alloys | Platinum, palladium, gold, silver, steel, copper and their alloys | Gold, silver, steel, copper and their alloys |
| Max capacity | 2kg platinum | 4kg platinum | 12kg gold / 6kg silver |
| Melting time | 2 minutes | 3 minutes | 5 minutes |
| Max temperature | 2800℃ | 2800℃ | 1800℃ |
| Dimension | 740*500*1360mm | 740*500*1360mm | 740*500*1360mm |
| Weight | 95 kg | 100 kg | 95 kg |
| Heating technology | IGBT Induction heating | IGBT Induction heating | IGBT Induction heating |
| Water pump | Equipped | Equipped | Equipped |
| Cooling way | Water cooling | Water cooling | Water cooling |
- Wide Applicability: Suitable for jewelry manufacturing, precious metal recycling, and jewelry processing, compatible with gold, silver, platinum, palladium, rhodium, iridium, ruthenium, and other precious metals.
- Ultra-High Temperature Performance: Capable of rapidly heating up to 2800℃ to meet the melting requirements of high-melting-point precious metals.
- Fast Melting: Able to melt 4–12kg of gold, silver, or platinum in just 2–5 minutes, greatly improving production efficiency.
- Precise Operation: Equipped with a tilting casting function for safe and accurate metal pouring.
- Efficient Cooling: Uses a reliable water cooling system to effectively reduce equipment temperature and ensure stable long-term operation.
- Safe and Reliable: Optimized design enhances operational safety, fully protecting operators during use.
- High-Quality Casting: Supports small-batch rapid processing, ensuring uniform melting and superior finished product quality.
Why SuperbMelt Precious Metal Furnace



Any Question About SuperbMelt Induction Furnace For Casting
A Comprehensive Guide to Precious Metal Furnaces
Introduction of mining of platinum
Precious metals such as gold, silver, platinum, palladium, and rhodium have been central to human culture, investment, and technology for centuries. The need to efficiently melt, refine, and cast these metals has driven the development of advanced furnaces. Understanding the history and modern applications of precious metal furnaces helps manufacturers, jewelers, and refiners select the right equipment for their needs.
1.1, Early Platinum Mining
The early human need to melt low-melting-point precious metals such as gold and silver gave rise to primitive tools centered on “fuel heating + basic temperature control.” Although simple, these tools laid the foundation for later precious metal processing and can be divided into three main types:
Clay Bonfire Furnaces (3000 BCE – 500 BCE):
In early civilizations such as ancient Egypt and Mesopotamia, semi-spherical furnaces were built using clay mixed with plant ash. Gold and silver ores or rough materials were placed inside, while wood or dry branches burned beneath as fuel. To increase temperature, small side holes were made in the furnace, and craftsmen used bellows to repeatedly blow air, raising the temperature to about 1000–1200℃. This barely met the melting requirements of gold (1064℃) and silver (961℃). However, there was no precise temperature control—craftsmen relied on flame color (e.g., “bright yellow flame” indicating gold’s melting point). Each batch could melt only a few dozen grams of metal, with over 15% loss, mainly for making simple jewelry and coin blanks.
Bronze/Iron Bellows Furnaces (500 BCE – 1500 CE):
During the Greek, Roman, and Chinese Qin-Han periods, materials improved with furnaces made of bronze or cast iron, offering stronger heat resistance. Charcoal, with higher combustion efficiency, replaced wood as fuel. A key advancement was the “double-action bellows,” where two wooden boxes alternately pumped air, ensuring continuous oxygen supply and a steady furnace temperature of 1200–1300℃. This allowed faster melting of gold and silver, as well as handling alloys with impurities (e.g., ancient “gold-copper alloys”). Ceramic crucibles began to be used to prevent contamination from furnace walls. Batch capacity increased to several hundred grams, though still inadequate for platinum (melting point 1772℃). Since the bellows required constant manual operation, efficiency remained limited by human labor.
Coke-Fired Reverberatory Furnaces (1500 CE – 1800 CE):
From the late Middle Ages to the pre-Industrial Revolution, advances in European jewelry and mining led to further upgrades. Coke, with higher calorific value, became the main fuel. The furnace was designed with a “reverberatory” structure—its sloped roof reflected heat onto the crucible, reducing heat loss and achieving 1400–1500℃. Adjustable air inlets allowed craftsmen to fine-tune airflow and temperature. Metal loss dropped to 8–10%, while batch size exceeded 1 kg, suitable for small-scale production such as religious vessels. However, temperature control still relied on manual judgment. In addition, coke combustion produced heavy smoke and dust, severely oxidizing metal surfaces and requiring additional polishing to remove oxide layers, which increased processing steps.
In summary, these early tools were limited by “low maximum temperature,” “poor temperature precision,” and “heavy reliance on manual labor and experience.” They were suited only for basic processing of low-melting-point precious metals like gold and silver. Not until the 19th century, with breakthroughs in electrical technology and industrial materials, were they gradually replaced by modern precious metal furnaces.
1.2, Modern Platinum Mining Techniques
After the Industrial Revolution, breakthroughs in electrical technology and advances in materials science drove a transformation in precious metal furnaces—from “fuel heating” to “precise temperature control.” Modern furnaces now focus on high-efficiency heating, precise control, and low-consumption environmental performance, forming a complete technological system that serves everything from jewelry workshops to aerospace-grade high-purity applications. Their core technological pathways fall into four categories:
1. Electromagnetic Induction Heating Technology: The Mainstream Choice for High-Efficiency Production
Electromagnetic induction is the dominant method in today’s precious metal processing. Its principle is to generate a magnetic field using high-frequency alternating current, inducing eddy currents inside the metal itself, which in turn heats and melts it—an “internal heating” process. IGBT (Insulated Gate Bipolar Transistor) modules serve as the core control unit, enabling precise adjustment of frequency and power for different conductive metals such as gold, silver, and platinum.
Key advantages:
Ultra-fast heating: Heat acts directly within the metal, reaching efficiencies of 60–70%, far above traditional fuel furnaces (<30%). For example, a 15kW medium-frequency induction furnace can melt 2kg of platinum in 2 minutes or 100g of gold in just 30 seconds—3–5 times faster than resistance furnaces.
Strong uniformity: The magnetic field creates an “electromagnetic stirring” effect, ensuring uniform distribution of temperature and alloy composition, crucial for precise alloys like 18K gold or platinum-rhodium.
Intelligent control: Using a PID closed-loop system combined with thermocouples and infrared sensors, temperature fluctuations can be kept within ±5℃. This prevents gold losses from overheating (reducing loss to below 0.5%) and ensures stable melting of platinum at 1772℃.
Applications include jewelry factories and precious metal recycling plants. Typical models range from Indutherm’s small induction furnaces (1–3kg) to SuperbMelt’s industrial-grade units (4–12kg), priced between $1,500 and $15,000, balancing efficiency and cost.
2. Resistance Heating Technology: The Preferred Option for Precision Processing
Resistance heating uses elements such as nichrome or silicon carbide to generate heat via electrical resistance. Heat is transferred to the crucible through radiation and conduction. Its strengths lie in temperature stability and ease of operation, making it ideal for small workshops.
Key features:
Precise temperature control: Smooth heating with minimal gradients. Though slower (melting 5kg of silver may take 1.5–2 hours), it is suited to processes requiring accuracy, such as annealing and small-batch alloying.
Clean and compact: No open flame or dust, with equipment sizes under 0.5m³, operable on standard mains electricity—perfect for jewelry studios and dental labs.
Cost-effective: Units cost $200–$2,000, with simple maintenance (only periodic element replacement).
Limitations: Heat efficiency is only 30–40%, and maximum temperatures usually stay below 1800℃, making it unsuitable for high-melting-point platinum-group metals like rhodium (1966℃) or iridium (2443℃).
3. Vacuum and Inert Gas Protection: The Core for High-Purity Processing
Platinum-group metals are highly prone to oxidation at high temperatures and often require ultra-high purity. Vacuum and inert-gas protection technologies address this need by isolating the metal from oxygen or creating controlled atmospheres to achieve “zero oxidation loss.”
Main approaches:
Vacuum Arc Remelting (VAR): In metallurgical-grade vacuums (0.1–13.3 Pa), the target metal serves as the electrode, melted by a high-temperature arc (>2000℃). This removes hydrogen, oxygen, and nitrogen impurities, while controlled solidification improves microstructure. Used in aerospace for high-purity platinum, tantalum, etc., typically requiring 1–3 remelts. Costs run $5,000–$50,000, with cycles lasting several hours.
Inert Gas Melting: A sealed chamber is filled with inert gases (e.g., argon), replacing air. This prevents oxidation at lower cost than VAR. For example, in dental labs, melting medical gold alloys under argon ensures biocompatibility by avoiding harmful oxides.
These methods achieve purities exceeding 99.99%, essential for high-end applications such as gold bonding wires in semiconductors and palladium catalysts in fuel cells.
4. Fuel Heating Technology: A Supplement for Emergency and Portable Use
Furnaces fueled by propane or natural gas, while limited in temperature precision, remain useful in specific scenarios due to their non-reliance on electricity.
Core advantages:
Portable and economical: Units cost only $100–$800, requiring no complex circuits. They are suitable for remote mines or field assays without electricity.
Drawbacks: Smoke and soot contaminate metals, temperature fluctuations can exceed ±50℃, losses reach 3–5%, and ventilation systems are needed for exhaust. Thus, they are suitable only for emergency or low-precision melting.
Technology Integration and Future Trends
Modern precious metal furnaces increasingly combine multiple technologies:
Industrial induction furnaces integrated with vacuum chambers enable “high-efficiency heating + oxidation-free melting.”
Small resistance furnaces paired with smart control panels balance convenience and accuracy.
Future development will move in three directions:
Energy efficiency – new insulation materials raising induction furnace efficiency above 80%.
Automation – AI-driven optimization of melting curves and predictive maintenance.
Customization – dedicated furnaces for specific metals, such as ultra-high-temperature designs for rhodium or corrosion-resistant crucibles for ruthenium.
These advances are driving precious metal processing from “experience-based” to “precision-controlled.”
1.3, Business value and investment market
The industrial application history of precious metal furnaces essentially reflects a mutually reinforcing process of “upgrading demand for precious metals” and “technological breakthroughs.” From large-scale industrial processing in the 19th century to high-precision applications in the 21st century, furnaces have evolved from “single-purpose melting tools” into “core equipment for full-process handling.” This evolution can be divided into four key stages, each corresponding to significant shifts in industry demand.
Stage 1: 1850–1900 — Mining-Driven “Batch Melting” Enlightenment
In the mid-19th century, global precious metal mining boomed (e.g., South Africa’s Witwatersrand gold mines, Nevada silver mines in the U.S.). Traditional hand-operated furnaces could not meet the demand for ton-scale crude metal processing, prompting the development of the first generation of industrial precious metal furnaces—coke-fired reverberatory furnaces.
Core application: Crude metal purification at mines. Gold extracted from ore (“barren gold” containing silver and copper impurities) was first melted to remove impurities. The reverberatory furnace, with its sloped structure and flame-reflecting design, could melt 50–100 kg of crude gold per batch at 1200–1300℃. Fluxes like borax absorbed impurities, raising crude gold purity from ~80% to over 95% before transport to refining plants.
Technical limitations: Coke combustion resulted in low heat efficiency (<25%) and SO₂ corrosion of the furnace. Temperature control was imprecise, with metal losses of 5–8%, and it could only handle gold and silver—not platinum, which had yet to be mined at scale.
Industry impact: For the first time, a “mine → furnace → refinery” production chain was realized, boosting global gold output from ~50 tons/year in 1850 to 200 tons/year by 1900, supplying the gold standard monetary system.
Stage 2: 1900–1950 — Industrial Demand Drives “Technical Diversification”
In the early 20th century, emerging industries like automotive and aerospace sharply increased demand for high-purity platinum (used in spark plug electrodes and turbine blades), while the jewelry sector began mass-producing karat gold (K gold) items. Single-purpose furnaces could not meet diverse requirements, spurring specialization in furnace technology.
Core applications:
Industrial platinum processing: In the 1920s, Germany’s Siemens developed electric arc furnaces, generating >3000℃ to melt platinum industrially. By the 1930s, vacuum arc furnaces were introduced to prevent oxidation, achieving 99.9% purity, suitable for aerospace platinum. Equipment cost exceeded $100,000 per unit, used only by large industrial enterprises.
Jewelry batch processing: In the 1940s, the U.S. company Inductotherm launched the first small medium-frequency induction furnace (5–10 kW), melting 1 kg of K gold in 10 minutes with 50% efficiency. Adjustable frequency controlled melting speed, ideal for “small-batch, multi-batch” jewelry production. Losses fell below 2%, rapidly replacing manual furnaces in the industry.
Industry impact: This period marked the first technical separation between industrial high-purity processing and consumer product batch production, expanding precious metals from monetary use to dual industrial and decorative applications. Furnaces became key linking equipment across sectors.
Stage 3: 1950–2000 — Electronics and Environmental Regulations Drive “Precision Upgrades”
After WWII, the electronics industry (gold in PCB wiring) and medical field (platinum in implants) demanded higher purity and precision, while stricter environmental regulations limited emissions from fuel furnaces. This drove a shift toward precise temperature control and clean heating.
Key technological breakthroughs:
IGBT induction technology (1970s): IGBT (Insulated Gate Bipolar Transistor) replaced traditional thyristors in induction furnaces, allowing precise current frequency control (50 Hz–10 kHz). For instance, Sumitomo’s IGBT induction furnace could control temperature within ±3℃, melt 100 g of gold in 20 seconds, and monitor molten levels in real time—perfect for “micro, high-precision” electronics applications.
Refined resistance furnaces (1980s): Silicon carbide elements replaced nichrome, raising maximum temperature to 1800℃. PID closed-loop control enabled 1℃ heating precision, suitable for dental gold alloys and laboratory alloy research. Compact designs (<0.3 m³) accommodated small spaces for high-precision work.
Environmentally upgraded fuel furnaces (1990s): Propane furnaces added secondary combustion and exhaust filtration, reducing CO emissions to <0.1%. Infrared temperature monitoring maintained fluctuations within ±20℃. These remained emergency backup units in remote mining areas.
Industry impact: Furnaces transitioned from single-function tools to integrated multi-process equipment, capable of melting, purifying, alloying, and annealing. Environmental design became standard, moving the industry from high-loss, high-pollution operations toward low-loss, clean processing.
Stage 4: 2000–Present — High-End-Driven “Intelligent and Custom” Maturity
In the 21st century, high-end sectors such as new energy (palladium for fuel cells) and semiconductors (gold bonding wires, platinum sputtering targets) require ultra-high purity (99.999%+) and micro-scale precision. The jewelry industry’s trend toward personalized customization also drove furnace technology toward intelligent and customized solutions.
Core applications:
High-purity industrial processing: Vacuum electron-beam melting furnaces produce 2500℃ for platinum, achieving 99.9995% purity and single-crystal growth for semiconductor targets. Costs exceed $1 million per unit. Plasma melting furnaces heat palladium to 5000℃ to form nano-scale powders for catalysts.
Personalized jewelry: Compact, intelligent induction furnaces (3–5 kW, ~20 kg) with touchscreen and mobile app control allow a single operator to melt, pour, and shape within 20 minutes. Up to 100 customized programs (e.g., 18K gold, Pt950) can be stored.
Refined precious metal recycling: Stepwise induction furnaces melt impurities at 800℃, then gold at 1200℃ and platinum at 1800℃, coupled with electromagnetic separation, achieving >99.5% recovery for e-waste and catalytic converters.
Industry impact: Furnace technology is deeply integrated into high-end manufacturing and circular economy, evolving from general-purpose to scenario-specific equipment. Intelligent designs (AI fault prediction, energy optimization) reduce operator dependency, moving precious metal processing from artisan skill to standardized, scalable production, further expanding applications.
From coke-fired reverberatory furnaces to vacuum electron-beam furnaces, the history of precious metal furnace applications reflects both continuous technological breakthroughs and the transformation of precious metals from rare resources into core industrial materials. Looking ahead, with developments in hydrogen energy, quantum chips, and other advanced fields, furnace technology will continue evolving toward ultra-high temperatures (>3000℃) and ultra-low-temperature melting to prevent lattice damage, sustaining innovation in high-end precious metal applications.
1.4, Key Advantage: Adapt to Ductile Precious Metals
One of the core values of precious metals such as gold, silver, and platinum lies in their extreme ductility—for example, 1 gram of gold can be drawn into a 400-meter fine wire, and 1 gram of platinum can be rolled into a 0.0001 mm thin sheet. This property imposes very high requirements on temperature stability, uniform heating, and controlled cooling during melting and processing. Modern precious metal furnaces, through targeted technological designs, precisely address the shortcomings of traditional tools that could damage ductility, becoming the core equipment for preserving and enhancing metal ductility. Their advantages can be summarized in three main dimensions:
1. Precise Temperature Control: Preventing Crystal Structure Damage
The ductility of precious metals depends on a uniform internal crystal structure. Excessive heat or large temperature fluctuations can fracture crystals or trap impurities, directly reducing ductility—for example, gold heated above 1200℃ can over-soften, causing breakage during subsequent drawing. Modern furnaces employ dual temperature control technologies to provide a stable thermal environment for high-ductility metals:
Real-time monitoring and dynamic adjustment: Furnaces are equipped with thermocouples (contact) and infrared sensors (non-contact) to monitor molten metal temperature, with feedback intervals of 0.5 seconds. Coupled with IGBT modules or PID control systems, power is adjusted in real time—for instance, when melting gold near its 1064℃ melting point, the system automatically reduces power to prevent overshoot (±5℃), avoiding coarse crystal growth.
Segmented temperature profiles for different metals: Dedicated heating curves are preset for metals sensitive to ductility. For platinum, ductility is most stable between 1600–1800℃; the furnace first rapidly heats to 1500℃, then rises at 5℃/min toward 1772℃, minimizing local thermal differences and ensuring uniform crystal growth.
This precise temperature control can preserve over 95% of a metal’s ductility. Gold processed in modern induction furnaces, for example, exhibits a 60% lower breakage rate during wire drawing compared with traditional fuel-fired furnaces.
2. Uniform Heating: Preventing Localized Ductility Variations
Traditional furnaces (e.g., coke-fired reverberatory furnaces) rely on flame conduction, often creating hot spots where the center is hotter than the edges. This leads to uneven molten metal, forming “soft-hard inconsistencies” after cooling—some regions retain ductility, while overheated areas become brittle, causing cracks during rolling or engraving. Modern furnaces ensure uniform heating through:
Electromagnetic induction (“internal heating”): Induction furnaces generate heat directly within the metal via a magnetic field, eliminating conduction gradients. For 18K gold, temperature differences across the molten bath can be kept within 3℃, producing alloys with consistent ductility during subsequent rolling.
Resistance furnace with radiation + stirring: Resistance furnaces, while relying on radiation, can be equipped with inert gas stirring (e.g., argon jets) to gently mix the molten metal, balancing regional temperatures. For high-ductility dental alloys (silver-palladium), induction plus argon stirring improves uniformity by 40%, producing crowns that better withstand occlusal stress.
Uniform heating keeps ductility variation within 5%, solving the traditional issue of uneven ductility within a single batch.
3. Controlled Cooling: Preventing Crystal Defects
The cooling rate of molten precious metals directly affects crystal reconstruction. Rapid cooling can cause undercooled structures, making metals brittle (e.g., silver loses ~30% ductility if cooled too quickly). Slow cooling reduces efficiency and increases oxidation risk. Modern furnaces employ gradient cooling technologies to protect ductility while maintaining efficiency:
Segmented cooling adapted to metal properties: Cooling rates are adjusted based on crystallization temperatures. For silver (melting point 961℃), the temperature is slowly lowered at 20℃/min from 900–800℃ to allow ordered crystal growth, then accelerated to 50℃/min from 800–500℃ for efficiency, achieving ductility retention above 92%.
Inert gas-protected cooling: For oxidation-prone metals like platinum and palladium, argon is continuously introduced during cooling, preventing air exposure while allowing controlled cooling. For platinum, argon flow is gradually reduced from 10 L/min to 2 L/min, combined with furnace insulation for slow, oxidation-free cooling, enabling rolling to 0.01 mm sheets.
Isothermal annealing (advanced feature): Some high-end furnaces maintain specific temperatures (e.g., 400℃ for gold, 600℃ for platinum) for 10–30 minutes during cooling to relieve internal stress and repair minor crystal defects, further enhancing ductility by 5–10%.
Furnaces as the Core Guardians of Ductility
High ductility distinguishes precious metals from ordinary metals and underpins their value in jewelry (fine engraving), electronics (ultra-fine wiring), and medical devices (implant deformation compatibility). Traditional melting tools, with uneven heating and uncontrolled cooling, often destroy this property. Modern precious metal furnaces, through precise temperature control, uniform heating, and controlled cooling, have shifted from “damagers” to protectors, not only fully preserving natural ductility but also enhancing performance through optimized processing. They have become indispensable core equipment for high-value precious metal processing.
What are the Precious Metal Melting Furnaces on the Market Nowadays
2.1, Vacuum electroslag platinum melting induction furnace
Vacuum electroslag precious metal furnaces leverage “ultra-high purification” and “performance optimization” as their core competitive advantages. Through the combination of vacuum protection + slag refining + directional solidification, they overcome challenges that conventional furnaces cannot—achieving high purity, low defects, and oxidation resistance. Although their cost and efficiency limit use in standard applications like jewelry processing, they remain irreplaceable equipment for high-value precious metal applications in aerospace, medical, and semiconductor industries. With iterative improvements such as low-fluoride slags and energy-saving control systems, operating costs are gradually decreasing, and future applications are expected to expand further into precious metal recycling and refining, such as recovery of platinum-group metals from retired aircraft engines.

2.2 Electromagnetic Induction Smelting Furnace for Precious Metals
Electromagnetic induction precious metal furnaces are mainstream equipment that achieve “internal heating” of precious metals based on the principle of electromagnetic induction. With their core advantages of high efficiency, precise temperature control, and clean low-consumption operation, they have replaced traditional fuel-fired and resistance furnaces, becoming essential equipment in jewelry processing, precious metal recycling, and industrial alloy preparation. Technically, these furnaces generate heat within the metal itself via a high-frequency alternating magnetic field, enabling efficient melting and refining of gold, silver, platinum-group metals, and alloys, suitable for scenarios ranging from gram-scale laboratory experiments to ton-scale industrial production.
1. Core Working Principle: Electromagnetic Induction Driven “Internal Heating”
The heating process in these furnaces requires no flame or resistive elements, relying entirely on direct conversion of electromagnetic energy into heat. The core principle can be broken down into three key stages, achieving contactless, high-efficiency melting:
Magnetic Field Generation and Frequency Conversion: The system uses IGBT (Insulated Gate Bipolar Transistor) or MOSFET inverter modules to convert mains AC power (50/60 Hz) into DC, then invert it into high-frequency AC (medium-frequency: 300 Hz–10 kHz; high-frequency: 30–100 kHz). High-frequency current flows through a copper induction coil surrounding the crucible, generating an alternating magnetic field whose intensity increases with frequency.
Eddy Current Heating and Metal Melting: When precious metal or alloy is placed in a refractory crucible within the magnetic field, the alternating field induces closed-loop eddy currents inside the metal. The electrical resistance generates Joule heat, providing “inside-out” uniform heating. Unlike traditional furnaces that rely on external conduction, this internal heating allows metals to rapidly reach melting points (gold 1064℃, platinum 1772℃), with maximum furnace temperatures up to 2000℃, suitable even for high-temperature platinum-group metals like rhodium.
System Coordination and Stable Temperature Control: The copper induction coil is water-cooled to prevent damage from high-frequency heating. Dual temperature monitoring with thermocouples and infrared sensors provides real-time feedback, and a PID closed-loop system dynamically adjusts power output to maintain ±5℃ temperature stability. Additionally, the alternating magnetic field generates mild electromagnetic stirring, ensuring uniform molten composition and temperature, laying a solid foundation for subsequent processing.
2. Key Technology Classification: Frequency-Adapted Applications
The primary differentiation among electromagnetic induction furnaces lies in current frequency, which determines heating efficiency and suitable application scenarios. The two main branches—high-frequency precision type and medium-frequency high-efficiency type—cover the entire chain from research to mass production:
| Technology Type | Core Parameters (Frequency / Power) | Heating Characteristics | Suitable Applications | Typical Advantages |
|---|---|---|---|---|
| High-Frequency Induction Furnace | 30–100 kHz / 0.5–15 kW | Localized, concentrated heating; extremely fast (1–3 min to melt 500 g platinum) | Laboratory R&D, teaching demonstrations, small-scale recycling (5–500 g per batch) | 1. Rapid heating reduces precious metal volatilization (loss ≤0.1%) 2. Small capacity suitable for micro-scale experiments, minimizing material waste 3. Flexible parameter switching; 5–10 comparative experiments per day |
| Medium-Frequency Induction Furnace | 300 Hz–10 kHz / 15–500 kW | Uniform heating, capable of long-duration full-load operation | Jewelry batch processing, industrial alloy production, large recycling plants (1–50 kg per batch) | 1. Thermal efficiency up to 60–70%, >50% more energy-efficient than resistance furnaces 2. Supports 24-hour continuous operation, meeting mass production needs 3. Local temperature adjustment via coil spacing, suitable for complex alloys |
2.3 Precious Metal Resistance Furnaces
Precious metal resistance furnaces generate heat through electrically powered resistive elements, transferring energy via conduction and radiation. Characterized by precise temperature control, stable operation, and ease of use, they occupy an irreplaceable role in laboratory R&D, small-batch fine processing, and annealing applications. Technically, these furnaces convert electrical energy into heat, applying controlled thermal transfer to precious metal materials. While their heating efficiency is lower than that of electromagnetic induction furnaces, they offer distinct advantages in temperature stability and operational safety, making them the preferred choice for small- to medium-scale processing and experimental environments.
Why Equipment is Needed to Melt Precious Metals
3.1 History of Use of Precious Metals
The history of precious metal applications—from decorative currency to high-end industrial materials—directly drove the development of specialized melting equipment. Fundamentally, this demand stems from the need for processing precision dictated by application scenarios:
Ancient Low-Precision Demand
From 3000 BCE to the 19th century, precious metals were primarily used for jewelry, ritual vessels, and currency. Purity requirements were only around 80%-90%, and batch sizes were small (from a few grams to a few kilograms). Simple tools such as clay pit furnaces or bronze bellows furnaces were sufficient. Artisans relied on experience to judge temperatures; although metal loss exceeded 15%, these methods met basic melting needs.
Example: Ancient Egyptians used charcoal furnaces to melt gold for pharaoh masks, requiring no precise temperature control—simply melting gold into liquid form was enough for casting.
Modern Industrial Demand
From the late 19th to mid-20th century, the rise of the automotive (platinum spark plugs) and electronics (gold wires) industries increased purity requirements to above 95% and demanded larger batches (tens of kilograms per run). Simple tools exposed limitations in temperature stability and efficiency: traditional coke furnaces fluctuated ±50°C, causing uneven platinum melting and failing to meet spark plug heat-resistance standards; manual bellows could only process 1–2 batches per day.
Industrial-grade resistive furnaces and coke reflection furnaces emerged, incorporating simple temperature control (e.g., manual air doors) and batch melting designs, enabling semi-automated precious metal processing and supporting early industrial needs.
Modern High-Precision Demand
From the 21st century onward, sectors such as new energy (palladium fuel cells), semiconductors (99.999% pure gold bonding wires), and medical devices (implant-grade platinum stents) require purity exceeding 99.99%, while also demanding uniform microstructure and zero oxidation loss. Ordinary equipment cannot meet these standards.
Examples:
Semiconductor-grade gold with 0.001% impurity can reduce chip conductivity by 30%.
Oxidized palladium powder in fuel cells can cause immediate failure.
Specialized furnaces equipped with vacuum protection and precise temperature control—such as vacuum induction or plasma furnaces—became the only viable solution. These technologies address the three core challenges of high purity, low loss, and stable performance, forming the foundation for modern precious metal applications.
In short: The evolution of precious metal applications from low to high standards transformed specialized melting equipment from an optional tool into a necessity. Without such equipment, high-end applications of precious metals would be impossible.
3.2 Source of Precious Metals
The natural sources and recycled materials of precious metals determine that their melting must rely on specialized equipment—raw ores have low purity and complex composition, while recycled materials contain numerous impurities. Ordinary equipment cannot achieve effective separation or low-loss extraction.
1. Natural Ores: Low Purity + Complex Impurities Require Specialized Refining
Precious metals in nature often exist as associated ores with very low purity and complex compositions, requiring specialized equipment for stepwise refining:
Extremely low ore grade: Gold content in raw ore is typically only 0.1–5 g/ton (0.00001%–0.0005%). Ore must first be concentrated into “gold concentrate” (10%–30% purity) using beneficiation equipment such as flotation machines or gravity separators, then melted in a specialized furnace to remove impurities.
Example: Using a medium-frequency induction furnace heated to 1200°C, fluxes such as borax absorb iron, silicon, and other impurities, increasing purity to 80%–90%, followed by electrolytic refining to achieve 99.99% pure gold. Ordinary metal furnaces (e.g., steel furnaces) cannot control temperature accurately, causing flux failure and incomplete impurity removal, yielding final purity of only 50%–60%, which is unusable.Complex associated metals: Platinum-group metals (PGMs) often coexist with copper and nickel (e.g., in South African platinum ore, the Pt:Cu+Ni ratio is ~1:1000). Specialized equipment enables stepwise separation: a vacuum electric slag furnace can first melt copper (1085°C) and nickel (1455°C) to remove them, then raise the temperature to 1772°C to melt platinum. Ordinary furnaces would melt everything together, causing metal mixing and lowering recovery. Specialized equipment ensures platinum recovery above 95%.
2. Recycled Materials: Diverse Composition + Variable Forms Require Adaptable Equipment
Recycled precious metals—such as old jewelry, scrap circuit boards, and catalytic converters—have even more complex compositions and forms (blocks, wires, powders). Ordinary equipment cannot handle them efficiently:
Complex composition: Scrap circuit boards mix gold with plastics, copper, aluminum, and low-melting metals such as lead and tin (melting point 232°C). Using a conventional furnace directly would burn plastics (producing toxic gases) and allow lead/tin to mix with gold, lowering purity. Specialized precious metal recovery furnaces (e.g., stepwise induction furnaces) can first heat to 800°C to burn off plastics and volatilize low-melting metals, then raise temperature to 1064°C to melt gold, using electromagnetic separation to remove copper and aluminum. This yields gold purity of 99.9% with recovery above 98%.
Variable forms: Recycled platinum jewelry may include rings, necklaces, platinum wires, and powders. Ordinary furnace crucibles cannot accommodate all forms: blocks may heat unevenly, fine powders may be lost to airflow. Specialized furnaces use anti-splash crucibles (with flow-guiding grooves) and negative-pressure collection systems. For example, when processing platinum powder, the negative-pressure system collects splashed particles, keeping loss below 0.5%, whereas ordinary furnaces can lose 5%–10%.
3.3 What Equipment is Needed to Process Precious Metals
Melting precious metals is not an isolated step, but part of a complete workflow: “pre-treatment – melting – forming – inspection.” Specialized melting equipment must work in coordination with other supporting devices to complete the full process, whereas ordinary equipment cannot integrate into this chain.
1. Pre-treatment Equipment: Clearing the Way for Melting
The efficiency of melting directly depends on the pre-treatment of raw materials (crushing, sorting, impurity removal). Pre-treatment equipment must match the input requirements of the furnace:
Crushing equipment: Large recycled precious metal items (e.g., scrap platinum vessels) must be crushed into 5–10 mm pieces using dedicated precious metal crushers made of wear-resistant tungsten steel to avoid contamination. Ordinary metal crushers (made of standard steel) may introduce steel impurities, requiring additional impurity removal during melting, increasing costs. Furthermore, ordinary crushers produce oversized fragments (20–30 mm), causing uneven heating and prolonging melting time by 50%.
Sorting equipment: Mixed precious metals (e.g., gold, silver, copper alloys) require spectroscopic sorting machines to quickly identify and separate metals before melting. For example, sorted gold is placed into a 1064°C furnace, silver into a 961°C furnace, preventing “cross-melting” that would reduce purity. Without sorting, the mixed melt must undergo secondary electrolytic refining, adding 1–2 days and increasing metal loss by 3%–5%.
The output standards of pre-treatment equipment (crushing size, sorting accuracy) must precisely match the input requirements of specialized furnaces to form an efficient “pre-treatment → melting” workflow, which ordinary equipment cannot achieve.
2. Forming and Inspection Equipment: Shaping + Quality Control
After melting, precious metals must be formed (casting, rolling) and inspected, which requires the furnace to provide melting parameters (temperature curves, hold times) to ensure precision:
Forming equipment: For example, a vacuum lost-wax casting machine for jewelry must adjust mold temperature (e.g., 800°C) according to furnace-provided parameters (platinum melt at 1772°C, hold time 5 minutes) to prevent cracks from pouring molten metal into a cold mold. Ordinary casting machines cannot receive parameter signals from the furnace and rely on experience, yielding only 60%–70% qualified products. Specialized equipment combined with furnace data can achieve over 95% pass rate.
Inspection equipment: Product purity is tested with X-ray fluorescence spectrometers, which compare results against furnace impurity-removal parameters (e.g., flux amount, melting temperature). For example, if the furnace records “melted at 1200°C with 20 g borax,” but the spectrometer detects excess iron, the furnace parameters can be adjusted to optimize the process, forming a closed-loop of inspection → feedback → optimization. Ordinary furnaces without data recording cannot interface with inspection equipment, making it impossible to trace the source of purity issues.
3.4 How to Choose a Quartz Crucible
Quartz crucibles are the core consumable in precious metal melting, directly affecting melting quality. Their selection must be precisely matched with the specialized furnace and type of precious metal, as ordinary crucibles cannot meet these requirements. This also indirectly underscores the necessity of specialized equipment, since such equipment relies on dedicated consumables to function optimally:
1. Material Compatibility: Avoid Contaminating Precious Metals
Ordinary quartz crucibles (SiO₂ purity 95%-98%) contain impurities like aluminum and iron, which can leach into the metal during melting. For example, melting gold in a standard crucible can introduce aluminum, darkening the gold and reducing its purity below 99.5%. In contrast, specialized precious metal crucibles (SiO₂ purity ≥99.9%, impurity <0.001%) ensure that the metal’s purity remains unaffected.
For platinum-group metals, which require extremely high temperatures (e.g., platinum melts at 1772°C), high-temperature-resistant quartz crucibles (resistant >2000°C) are necessary. Ordinary crucibles (resistant up to 1500°C) will soften and deform at 1772°C, causing molten metal leaks, safety hazards, and material loss.
2. Size and Shape Matching: Fit Furnace and Melting Requirements
The crucible’s size must match the furnace’s chamber and the batch size. For instance, the SuperbMelt SPB-TB2 furnace (melting 2 kg of platinum per batch) requires a 2.5–3 L crucible (leaving 20%-30% free space to prevent overflow). Using an ordinary 5 L crucible disperses heat, doubling melting time. Additionally, standard flat-bottom crucibles prevent full utilization of the furnace’s electromagnetic stirring, reducing melt uniformity by 40% and affecting subsequent processing.
3. Pre-treatment Requirements: Improve Melting Efficiency
Specialized crucibles must be preheated at high temperatures (e.g., 1000°C for 30 minutes) to remove moisture and surface impurities, preventing bubble formation in the melt. Ordinary crucibles, used without preheating, can cause gas bubbles in the molten metal, producing porosity in castings and increasing scrap rates by 15%-20%.
Conclusion
1. What is a precious metal refinery?
1. Core Functions: From Impurity Removal to Value Upgrade
- Impurity Removal (Purification)Raw precious metals (e.g., gold ore concentrates with 10%-30% purity, recycled jewelry scrap with metal alloys) contain non-precious impurities (iron, copper, lead) and non-metallic substances (silicon, oxides). Refineries use physical (melting, filtering) and chemical (acid leaching, electrolysis) methods to eliminate these impurities. For example, gold with 80% purity can be refined to 99.99% pure gold through electrolysis, which removes even trace impurities (as low as 0.001%).
- Metal Separation (Segregation)Precious metals are often mixed together in raw materials—e.g., platinum-group metals (platinum, palladium, rhodium) coexist in mineral ores, and old electronic scrap contains both gold and silver. Refineries use temperature-controlled melting (different metals have different melting points) or chemical reactions (specific reagents react only with certain metals) to separate them. For instance, in recycling automotive catalytic converters, refineries first melt the scrap at 1600°C to separate platinum-palladium-rhodium from ceramic carriers, then use acid to dissolve and isolate each metal individually.
- Forming Standardized ProductsPurified precious metals are processed into standardized forms to meet downstream needs. Common products include:
- Ingot/bars (1kg, 10kg, etc.) for jewelry manufacturing and investment;
- Fine powders (nanoscale to micrometer-scale) for fuel cell catalysts and electronic paste;
- Wires or sheets for semiconductor bonding wires and medical implants. These products must meet international standards (e.g., LBMA “Good Delivery” for gold bars) to ensure consistency and marketability.
- Recycling and Resource ReuseA large part of a refinery’s raw materials come from recycled sources: old jewelry, discarded electronics (circuit boards, chips), spent industrial catalysts, and laboratory waste. Refineries recover precious metals from these “waste materials,” reducing reliance on mineral mining and promoting circular economy. For example, 1 ton of discarded mobile phones can yield 300g of gold, 3kg of silver, and 100g of palladium—refineries extract these metals efficiently, with recovery rates often exceeding 95%.
2. Key Equipment: The “Tools” Behind Refining
- Melting & Heating EquipmentThese devices provide controlled high temperatures for impurity removal and metal separation, including:
- Electromagnetic induction furnaces (for batch melting of gold/silver, with temperature accuracy ±5°C to avoid metal volatilization);
- Vacuum electroslag furnaces (for refining platinum-group metals, using vacuum to prevent oxidation and ensure high purity);
- Plasma furnaces (for ultra-high-temperature melting of refractory metals like rhodium, with temperatures up to 5000°C).
- Chemical Processing EquipmentThese are used for chemical purification and metal separation, such as:
- Electrolytic cells (for electrolytic refining of gold/silver—pure metal is deposited on the cathode, while impurities remain in the electrolyte);
- Acid leaching tanks (for dissolving impurities or specific metals—e.g., using aqua regia to dissolve gold from electronic scrap, leaving other metals unreacted);
- Precipitation reactors (for isolating target metals from solutions—e.g., adding reducing agents to gold-containing acid solutions to precipitate pure gold powder).
- Detection & Quality Control EquipmentTo ensure product purity meets standards, refineries use high-precision detection tools:
- X-ray Fluorescence (XRF) spectrometers (for rapid on-site analysis of metal content, with detection limits as low as 0.001%);
- Inductively Coupled Plasma Mass Spectrometers (ICP-MS) (for laboratory-level trace impurity testing, capable of detecting elements at the ppb level—1 part per billion);
- Density meters (for verifying gold/silver purity through density measurements, a quick supplementary testing method).
2. How long does it take to melt gold in a furnace?
3. How much gold is lost when smelting?
1. Key Factors Determining Gold Loss
- Physical Volatilization: Gold has a low vapor pressure at its melting point (1064°C), but high temperatures (exceeding 1200°C) or prolonged heating will increase its volatilization. For example, if the furnace temperature is accidentally raised to 1300°C, volatilization loss can double compared to the standard 1064°C.
- Chemical Oxidation/Reaction: Gold is chemically stable, but impurities in raw materials (e.g., copper, iron, sulfur) can react with oxygen or fluxes (e.g., borax) at high temperatures, forming compounds that “entrain” tiny gold particles into slag (the impurity layer), leading to loss.
- Operational Errors: Spills during pouring molten gold, residual gold stuck to the crucible, or incomplete separation of gold from slag due to improper stirring can all cause avoidable losses.
2. Typical Gold Loss Rates by Scenario
| Smelting Scenario | Typical Loss Rate | Key Reasons for Loss |
|---|---|---|
| Industrial Smelting (SuperbMelt Induction Furnace) | 0.1%–0.5% | Advanced electromagnetic induction heating (precise temperature control ±5°C, minimal volatilization); equipped with slag separation and crucible cleaning designs to reduce residual loss. |
| Jewelry Factory Smelting (Medium-Frequency Induction Furnace) | 0.5%–1.5% | Processes alloyed gold (e.g., 18K gold) with more impurities; occasional small spills during batch pouring (e.g., for jewelry casting) contribute to minor losses. |
| Small-Scale Recycling (Propane Fuel Furnace) | 2%–5% | Open-flame heating (temperature fluctuations ±50°C, higher volatilization); simple slag separation (incomplete impurity removal); crucibles may have more residual gold. |
| Outdated Equipment (Traditional Coke Furnace) | 5%–12% | Poor temperature control (often exceeding 1300°C); high pollution (impurities react more with gold); no professional slag treatment, leading to significant entrainment loss. |
- Use Precision Temperature-Controlled Equipment: Choose electromagnetic induction furnaces (e.g., SuperbMelt models) with PID temperature control to keep the temperature stable at 1064–1100°C (avoiding overheating and volatilization).
- Optimize Flux Usage: Add appropriate fluxes (e.g., borax, sodium carbonate) at a ratio of 2%–5% of the gold weight. Fluxes help melt impurities into slag and prevent gold particles from being trapped—too little flux causes incomplete slag formation, while too much wastes resources.
- Clean Crucibles and Equipment:
- Improve Slag Treatment: After smelting, cool the slag and crush it, then use a gravity separator or chemical leaching to recover tiny gold particles entrained in the slag—this
4. What are the five most precious metals?
1. Rhodium (Rh) – The “Most Valuable Precious Metal”
- Key Traits: A member of the platinum-group metals (PGMs), rhodium has a silvery-white appearance, an extremely high melting point (1966°C), and exceptional resistance to corrosion and high temperatures. It is also a critical catalyst for chemical reactions.
- Scarcity: Its global annual production is only about 30–40 tons (less than 1% of gold’s annual output), with over 80% coming from South Africa’s platinum mines (it is rarely found in independent deposits, mostly coexisting with platinum and palladium).
- Value Drivers: The largest demand comes from the automotive industry—rhodium is a core component of catalytic converters for gasoline vehicles, reducing nitrogen oxide emissions. It is also used in jewelry plating (e.g., “rhodium-plated white gold” for scratch resistance) and the electronics industry (for high-performance electrical contacts). Its market price is often the highest among precious metals, sometimes exceeding $20,000 per ounce.
2. Platinum (Pt) – The “Industrial Versatile Metal”
- Key Traits: Another PGM, platinum has a dense silvery-white texture, a melting point of 1772°C, excellent ductility, and biocompatibility (non-toxic to the human body).
- Scarcity: Global annual production is around 180–200 tons (about 5% of gold’s output), with South Africa (70%) and Russia (20%) being the main producers. It is extracted from ore at a low rate—typically 3–5 grams of platinum per ton of ore.
- Value Drivers: It is widely used in three core fields: automotive catalytic converters (for diesel vehicles), the jewelry industry (e.g., “platinum 950” jewelry), and healthcare (implantable devices like pacemaker electrodes and dental crowns). It also plays a role in fuel cells (as a catalyst for hydrogen oxidation), making it critical for the green energy transition.
3. Gold (Au) – The “Eternal Store of Value”
- Key Traits: Gold has a distinctive yellow luster, a melting point of 1064°C, extreme ductility (1 gram can be drawn into a 400-meter wire), and chemical inertness (it does not rust or corrode).
- Scarcity: Global annual production is about 3,000 tons, with major producers including China, Australia, and Russia. All gold mined in human history would fit into a cube roughly 22 meters on each side.
- Value Drivers: Unlike other precious metals, gold’s value comes from both industrial use and its role as a “safe-haven asset”:
- Industrial: Electronics (gold bonding wires for chips, corrosion-resistant coatings), healthcare (dental fillings, cancer treatment drugs).
- Financial: Central bank reserves, investment products (gold bars, ETFs), and traditional jewelry (accounting for ~50% of global demand). Its price is stable and widely recognized globally, making it a universal store of value.
4. Palladium (Pd) – The “Green Energy Catalyst”
- Key Traits: A PGM with a silvery-white color, a melting point of 1554°C, and strong catalytic activity—especially for hydrogen-related reactions. It is softer than platinum and more prone to oxidation at high temperatures.
- Scarcity: Global annual production is about 200–250 tons (similar to platinum), with Russia (40%) and South Africa (35%) as the main sources. Like rhodium, it is mostly a byproduct of platinum or nickel mining.
- Value Drivers: The automotive industry is its largest consumer (70% of demand)—palladium catalytic converters are used in gasoline vehicles (replacing rhodium in some cases due to cost). It is also critical for fuel cells (as a catalyst for hydrogen fuel cells) and the electronics industry (multilayer ceramic capacitors). In recent years, demand for green energy has pushed its price to near gold levels.
5. Iridium (Ir) – The “High-Temperature Resistant Champion”
- Key Traits: The densest PGM (density 22.56 g/cm³), with an extremely high melting point (2443°C)—the second-highest among all metals. It is highly resistant to corrosion, even by aqua regia (a mixture of nitric and hydrochloric acid that dissolves gold).
- Scarcity: The rarest of the five, with global annual production of only 3–5 tons (less than 0.2% of gold’s output). It is almost exclusively a byproduct of platinum mining in South Africa.
- Value Drivers: Its main use is in high-temperature industrial scenarios:
- Aerospace: Rocket engine nozzles, turbine blades for jet engines (resisting extreme heat and wear).
- Electronics: High-temperature thermocouples (for measuring temperatures up to 2000°C) and spark plugs for high-performance engines.
- Healthcare: Radioactive iridium-192 is used in cancer brachytherapy (treating tumors with localized radiation). Its scarcity and specialized industrial applications make it one of the most valuable precious metals.
5. What is a metal furnace?
A metal furnace is a device used to heat, melt, or refine metals for various industrial, laboratory, or manufacturing purposes. It provides a controlled environment where metals can reach their melting points or be processed under specific conditions such as temperature, atmosphere, or pressure.
Key points about a metal furnace:
Purpose:
Melting metals for casting, forging, or alloying.
Heat-treating metals to alter their properties (hardness, strength, ductility).
Purifying metals by removing impurities.
Types of Heating:
Fuel-based: Uses gas, coal, or oil to generate heat.
Electric: Includes resistance furnaces (heating elements) and induction furnaces (magnetic induction).
Specialized furnaces: Vacuum, plasma, or electron-beam furnaces for high-purity or high-temperature applications.
Applications:
Jewelry making, coin minting, and metal art.
Industrial alloy production, automotive parts, and aerospace components.
Precious metal recovery and refining.
In short, a metal furnace is the core equipment that transforms raw or recycled metals into usable forms under controlled thermal conditions.
6. What is the difference between a foundry and a furnace?
1. Furnace
Definition: A device or equipment used to generate heat for melting, heating, or refining metals.
Function: Provides the controlled environment to bring metals to their melting point or specific processing temperature.
Focus: The heating/melting step only.
Examples:
Electric induction furnace
Resistance furnace
Fuel-fired furnace
2. Foundry
Definition: A facility or workshop where metal casting is carried out, including melting, pouring, and forming metal products.
Function: A foundry is the whole production site for casting metals into shapes (ingots, parts, molds).
Scope: Includes furnaces, molds, pattern-making, finishing, and sometimes quality testing.
Examples:
A jewelry foundry producing gold pendants
An industrial foundry casting engine parts
Key Difference:
Furnace: Only heats or melts metal.
Foundry: Uses furnaces as part of a full system to cast and produce metal products.
Think of it this way: the furnace is the oven, the foundry is the kitchen. The furnace melts the metal; the foundry turns it into finished parts.











 © Copyright 2008-2026 Superb Electromachinery Co., Limited
© Copyright 2008-2026 Superb Electromachinery Co., Limited